Researchers Decode Molecular Mechanism to Prevent Cancer Using VEGFR1 Enzyme
Researchers have uncovered the molecular mechanism by which a cell surface receptor, belonging to a family of enzymes that bind growth factors, regulates key cellular functions and prevents cancers. This breakthrough, focused on an enzyme called VEGFR1, offers promising avenues for developing treatments for colon and renal cancers by stabilizing the inactive state of VEGFR1.
VEGFR1, an enzyme in the Receptor Tyrosine Kinases (RTK) family, remains autoinhibited in the absence of a ligand such as hormones. This self-inhibition prevents unnecessary cell differentiation, proliferation, survival, metabolism, and migration, which are processes often exploited by cancer cells.
RTKs, crucial cell surface receptors, convert extracellular signals from growth factors (ligands) into regulated cellular responses. Ligand binding activates intracellular enzymes (tyrosine kinases), which then add phosphate groups to tyrosine molecules. These molecules assemble a signaling complex that regulates various cellular functions, including cell growth and immune responses. Malfunctions in this process are linked to cancers, diabetes, and autoimmune disorders.
The VEGFR family, particularly VEGFR1 and VEGFR2, regulates blood vessel formation, essential for embryonic development, wound healing, tissue regeneration, and tumor growth. Targeting VEGFRs can treat both malignant and non-malignant diseases.
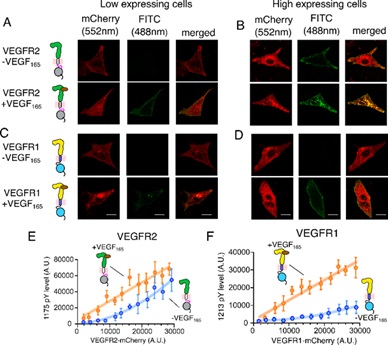
Researchers noted that VEGFR2 could be spontaneously activated without its ligand, while VEGFR1 remains autoinhibited even when overexpressed. VEGFR1 binds to its ligand VEGF-A with ten times higher affinity than VEGFR2, but this binding only triggers a transient kinase activation. VEGFR1 activation is linked to cancer-associated pain, tumor cell survival in breast cancer, and migration of colorectal cancer cells.
Dr. Rahul Das and his team at the Indian Institute of Science Education and Research (IISER), Kolkata, discovered an ionic latch unique to VEGFR1 that keeps it autoinhibited. This latch hooks the juxtamembrane segment onto the kinase domain, stabilizing VEGFR1's inactive state.
The researchers proposed that cellular tyrosine phosphatase plays a crucial role in modulating VEGFR1 activity. Their work, supported by DST-FIST facilities at IISER Kolkata, highlighted that phosphatase modulators could regulate pathological angiogenesis (formation of new blood vessels) in cancers.
This discovery, published in Nature Communications, suggests that small molecules targeting VEGFR1's autoinhibited state could effectively treat cancers where VEGFR1 is overexpressed, such as colorectal carcinoma and renal cancer. By stabilizing the inactive form of VEGFR1, new therapeutic interventions could prevent the spontaneous activation of VEGFR signaling, opening new avenues for cancer treatment.
This significant advancement underscores the potential of molecular research in developing precise cancer therapies, offering hope for more effective treatments in the future.