Import of Refurbished Medical Devices circumventing law into India Raises Alarming Concerns
In what could have enormous effect on the health of the patents in the country, a recent public interest litigation (PIL) in the Delhi High Court has raised significant concerns about the import of refurbished medical devices into India. The Patient Safety and Access Initiative of India Foundation (PSAIIF) filed the PIL, highlighting the major regulatory violations and public health risk these imports pose. In light of the increasing number of refurbished devices, including surgical robots, MRI machines, and CT scanners, entering the Indian market without proper approval, experts are advocating for stricter regulations and enforcement to safeguard the safety of patients and healthcare providers.
The PIL highlights weaknesses in India's regulatory framework that allow this refurbished equipment to avoid key approvals, creating a risk in important medical contexts. PSAIIF asserts that the increasing importation of reconditioned equipment that does not adhere to these laws undermines the Medical Device Rules of 2017, which set high safety and efficacy criteria for medical devices in India.
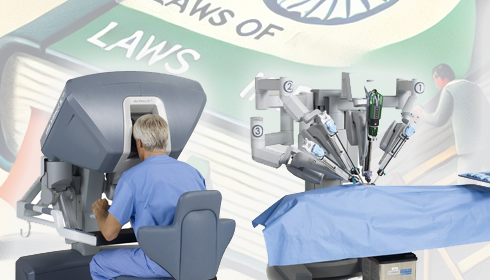
The petition focuses on the illegal import of medical devices, specifically high-end and high-value (HEHV) used equipment, without the necessary clearances from the Ministry of Environment, Forestry, and Climate Change (MoEFCC), particularly for hazardous waste management. This issue endangers patient safety while also undermining the "Make in India" strategy, which promotes domestic medical device production. The Federation of Indian Chambers of Commerce & Industry (FICCI) and other associations, such as AiMeD, have expressed strong opposition to these imports and advocated for government action. The PIL raises a major concern about the safety of these refurbished devices, which may not meet the rigorous performance and safety standards necessary for new equipment. According to PSAIIF, critical equipment imported without MoEFCC certification includes surgical robotics, CT scanners, and MRI machines. These devices are critical for diagnosing and treating patients. Hospitals and clinics are at risk of deploying potentially unreliable equipment due to a lack of regulatory oversight and endangering lives.
Professor Bejon Kumar Misra, the founder of PSAIIF, raised his concerns, stating that importing reconditioned medical equipment without the requisite certifications poses a direct threat to patient safety. To ensure that our healthcare system uses only certified and safe technology, the government must take action.
The petition alleges that Intuitive India Pvt. Ltd. imported reconditioned medical equipment worth over ₹250 crore without MoEFCC clearances since 2019. In 2019, the MoEFCC's report from its 133rd meeting in May 2024 allowed the company to import only one repaired Da Vinci X robot. However, it continued to import more devices in succeeding years without receiving approval. The lack of approval highlights a regulatory vacuum in monitoring and controlling the entry of used medical devices.
The Delhi High Court rejected the PIL on May 30, 2024, allowing PSAIIF to raise concerns with the defendants, which included the MoEFCC, DGHS (Directorate General of Health Services), and Customs officials. The court allowed PSAIIF to return if the responses from these entities were inadequate. PSAIIF has since received comments from both the MoEFCC and the DGHS; however, it has declined to take any further action until it receives legal advice.
Although the MoEFCC revised the Hazardous and Other Wastes (HOWM) Rules in 2016 to allow for the importation of high-end medical equipment under rigorous criteria, there are still worries regarding importers' lack of compliance. According to the rule, the exporting nation must offer substantial assistance, and imported equipment must have a residual life of at least seven years. However, the PSAIIF petition asserts that regular violations of these requirements lead to inadequate control over refurbished equipment entering the Indian healthcare system.
Furthermore, Professor Misra voiced concern about the negative impact of this clandestine importation on indigenous firms. He stated: "This inconsistency undermines the government's 'Make in India' initiative, threatening the confidence of domestic manufacturers and resulting in an 'Un-make in India' campaign." The unfettered entry of refurbished devices from outside discourages investment in local production, despite the fact that the DGHS encourages the importation of medical devices when equivalent domestic products are unavailable.
The PIL underscored the pressing necessity for more stringent controls and oversight in the importation of reconditioned medical devices. Although the MoEFCC has launched actions against enterprises involved in illicit imports, experts agree that a comprehensive policy framework is required to protect patient safety. More stringent import licence inspection, the enforcement of Medical Device Rules, and the development of clear criteria are necessary to ensure that reconditioned devices meet performance and safety standards before their use in India's hospitals.
As the case progresses, public health professionals and industry leaders are hopeful that the government will take immediate steps to ensure the safety, reliability, and self-sufficiency of India's healthcare system.